Causes of SIADH
Cancer. Tumors are the most common condition associated with SIADH. Lung tumors (small cell type) and head and neck tumors are the two tumor types most frequently associated with this syndrome. It has been noted that the lungs of patients with small cell cancer synthesize and secrete vasopressin.1
Nonmalignant lung disease has also been associated with SIADH. The pathophysiology, decreased oxygen, and increased CO2 that increase plasma vasopressin may decrease systemic resistance. This process impairs water clearance and may lead to dilutional hyponatremia.1
Medication use. Antipsychotic drugs, anticancer agents, antidepressants, anticonvulsants, narcotics, sulfonylureas, and angiotensin-converting enzyme inhibitors are among the various classes of drugs that can cause SIADH by causing inappropriate release of vasopressin.1,2
CNS disorders. The last group of conditions that cause SIADH comprises CNS disorders, such as trauma, infection, and hydrocephalus. These conditions are caused by alteration of the usual signaling pathway from the hypothalamus and brainstem, which regulate pituitary release of vasopressin.1
The role of vasopressin in hyponatremia in SIADH
Changes in sodium in SIADH can be attributed to an increase in vasopressin. The diagnosis of SIADH is made in the context of hyponatremia with plasma hypotonicity; urine osmolality exceeding plasma osmolality; elevated urinary sodium excretion despite normal salt and water intake; absence of edema or volume depletion; and normal renal, adrenal, and thyroid function.1
Several factors explain the changes in sodium excretion seen in SIADH3:
- Decreased aldosterone secretion secondary to increased extracellular fluid volume
- Increased filtered sodium as a result of increased glomerular filtration rate (GFR)
- Suppressed sodium resorption in the proximal tubules
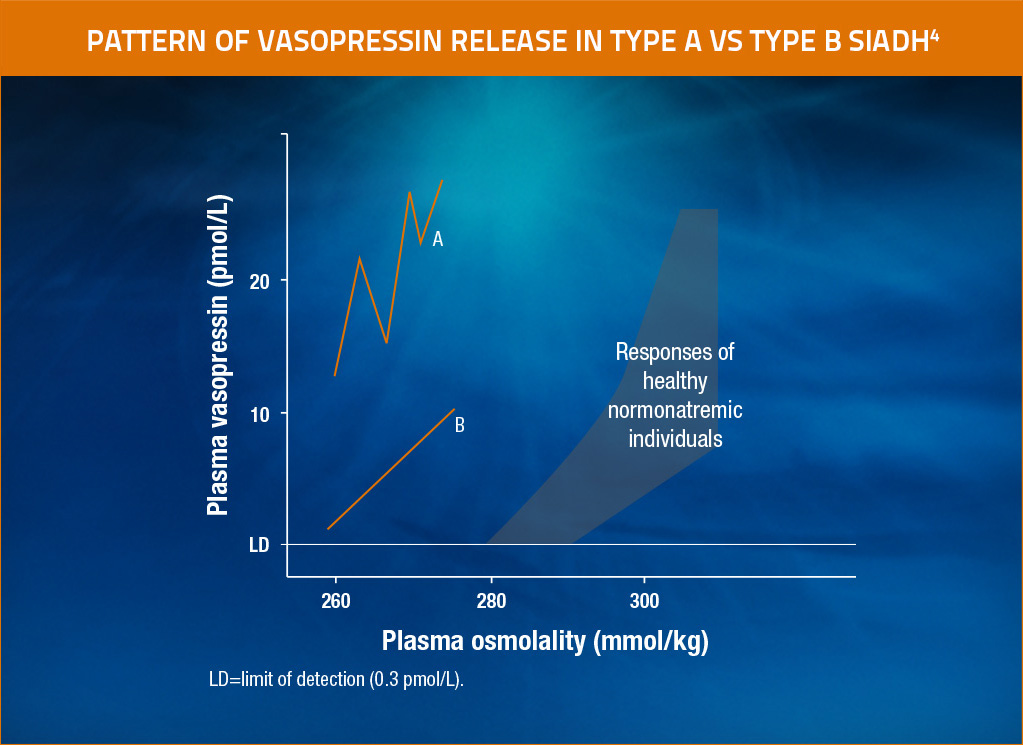
Different patterns of vasopressin release in SIADH
Type A SIADH4
- Found in approximately 40% of SIADH cases
- Excessive and erratic vasopressin release unrelated to serum osmolality
- Ectopic production of vasopressin by tumor tissue may account for this type of SIADH
Type B SIADH (also designated reset osmostat)4
- Vasopressin response to changes in serum osmolality is preserved
- Urine-diluting capacity is intact
- Osmotic threshold for vasopressin release is lowered
In type A SIADH, vasopressin release has no linear relationship to plasma osmolality. In type B SIADH, vasopressin release has a linear relationship to plasma osmolality, but the threshold is lower than normal. Adapted from Raftopoulos, Support Care Cancer, 2007.4

The SAMSCA MOA video illustrates the relationship between vasopressin and hyponatremia, as well as the potential benefits of vasopressin V2-receptor antagonism.