Hyponatremia is one of the most common electrolyte disorders associated with cancer1,2
In three large studies of cancer patients with hyponatremia, the rates of hyponatremia were3-6:
4.2%4
- n = 6766 patients at admission
- HN defined as a serum sodium level of <130 mEq/L
10.8%5
- n = 6612 patients at admission
- HN defined as a serum sodium level of <135 mEq/L
47%6
- n = 3357 patients
- 23% at admission
- 24% acquired during hospitalization
- HN defined as a serum sodium level <135 mEq/L
Not all of these patients will be appropriate for therapy with SAMSCA.
Hyponatremia is most often caused by SIADH in cancer patients1,7
- SIADH is most commonly found in patients with small cell lung cancer (SCLC)3
- SIADH has also been reported in patients with head and neck cancer3
- SIADH in patients with cancer may be due to3:
- Inappropriate secretion of vasopressin with malignancy
- Agents commonly used in cancer treatment and palliative care
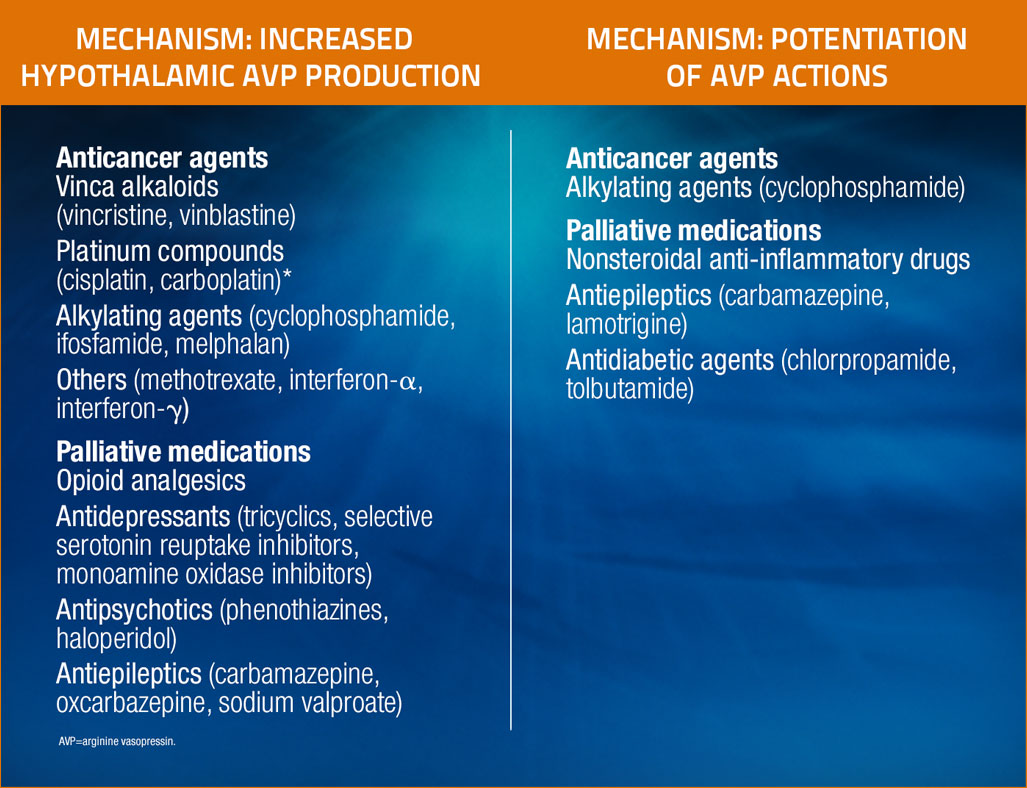
Certain drugs commonly used in patients with cancer are known to cause hyponatremia by inducing SIADH3
Adapted from Castillo et al, Oncologist, 2012.3
* Cisplatin may also cause hyponatremia by damaging renal tubes and interfering with sodium reabsorption.
Adapted from Castillo et al, Oncologist, 2012.3
Fluid restriction is considered the standard of care for dilutional hyponatremia8,9
Fluid restriction addresses total body water imbalance8,9
- In some patients, hyponatremia can resist correction with fluid restriction10
Fluid Restriction can be difficult in the oncology setting3
- Some patients with hyponatremia in SIADH experience an exaggerated need to drink due to downward resetting of the osmotic threshold for thirst11
The role of hyponatremia in SIADH in oncology