Treatment with SAMSCA® (tolvaptan)
SAMSCA is indicated for clinically significant hypervolemic and euvolemic hyponatremia: Serum sodium <125 mEq/L or less marked hyponatremia that is symptomatic and has resisted correction with fluid restriction
Our patient selection guide can help you identify appropriate patients for SAMSCA therapy.
Important steps in the treatment of patients with hyponatremia is to determine whether their disease is acute (defined as duration of <48 hours) or chronic (defined as duration of ≥48 hours),1 and to take into account the rate of serum sodium correction.2 The risk of hyponatremia-associated complications then must be balanced against the risk of serum sodium correction. Factors that should be considered before treating include the rapidity of onset of hyponatremia; degree, duration, and symptomatology of hyponatremia; and the presence or absence of risk factors for neurologic complications.1
Treatment of Acute vs Chronic Hyponatremia
Because of the different effects of acute versus chronic hyponatremia on the brain, the treatment strategy for each will differ. Tap on the disease states below to see treatment options.
Patients requiring an urgent need to raise serum sodium to prevent or treat serious neurological symptoms should not be treated with SAMSCA.
Limit duration of therapy to 30 days.
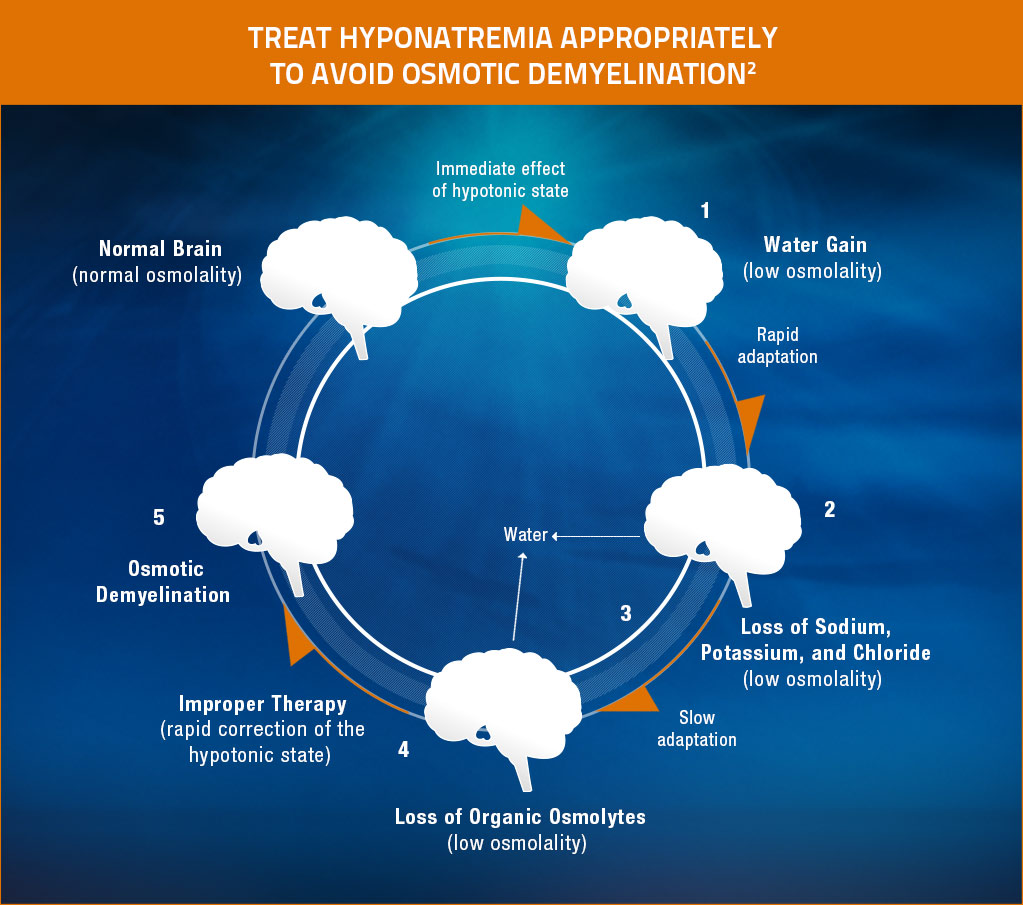
Effects of hyponatremia on the brain and adaptive responses
The brain’s adaptation process during hyponatremia may be associated with the rare but serious consequence of osmotic demyelination syndrome (ODS) if the rate of correction of sodium is too-rapid. ODS can develop 1 to several days after aggressive treatment of hyponatremia (e.g., too-rapid correction), even with water restriction alone.3
Adapted from Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589. Copyright © 2000 Massachusetts Medical Society. All rights reserved.
- Hypotonic hyponatremia results in water entering the brain.3
- Water gain leads to cerebral edema, intracranial hypertension, and a risk of brain injury.3
- Within hours, however, solutes exit the brain tissues, inducing loss of water.3
- This loss of water relieves the swelling of the brain. This adaptation process helps explain why even patients with severe hyponatremia may have few symptoms if the condition develops slowly.3
- Rapid correction of the hypotonic state can lead to shrinkage of the brain. Shrinkage of the brain leads to demyelination of pontine and extrapontine neurons, which can cause neurologic dysfunction in the form of quadriplegia, pseudobulbar palsy, seizures, coma, or even death. Patients with potassium depletion and malnutrition are at increased risk of this complication.3
Expert recommendations to avoid ODS in the management of hyponatremia
Experts advise that correction should be of an appropriate rate to reverse the manifestation of hypotonicity, but not to impose a risk of ODS.3 According to the expert panel recommendations for treating hyponatremia, the rate of correction of hyponatremia should be limited to <10 to 12 mEq/L in 24 hours and <18 mEq/L in 48 hours.2,3
As always, physicians must use their clinical judgment when treating patients with hyponatremia.

Review clinical cases about patients with hyponatremia in heart failure and hyponatremia in SIADH