Vasopressin is synthesized in the hypothalamus and secreted from the pituitary gland
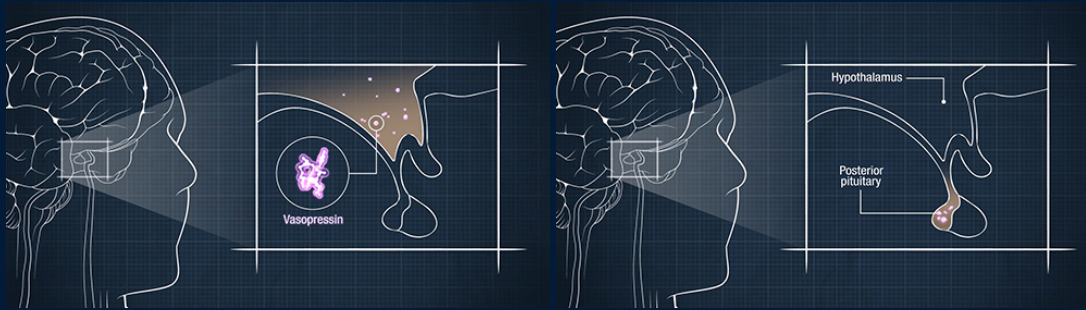
Vasopressin, also known as antidiuretic hormone, is a 9-amino-acid peptide that is synthesized in the nerve bodies in the supraoptic and paraventricular nuclei of the hypothalamus and is secreted from the posterior pituitary gland.1
Vasopressin is released in response to decreased plasma volume and increased serum osmolality2

Secretion of vasopressin is normally stimulated by increased plasma osmolality via activation of osmoreceptors in the anterior hypothalamus and by decreased blood volume or pressure via activation of baroreceptors in the carotid sinus, aortic arch, cardiac atria, and pulmonary venous system.1,3
The primary role of vasopressin is to control water balance, electrolyte balance, and blood pressure

Vasopressin controls the body’s water and electrolyte balance, and blood pressure through its antidiuretic effects on the kidney, resulting in decreased plasma osmolality, and/or increased arterial circulating volume.3,4
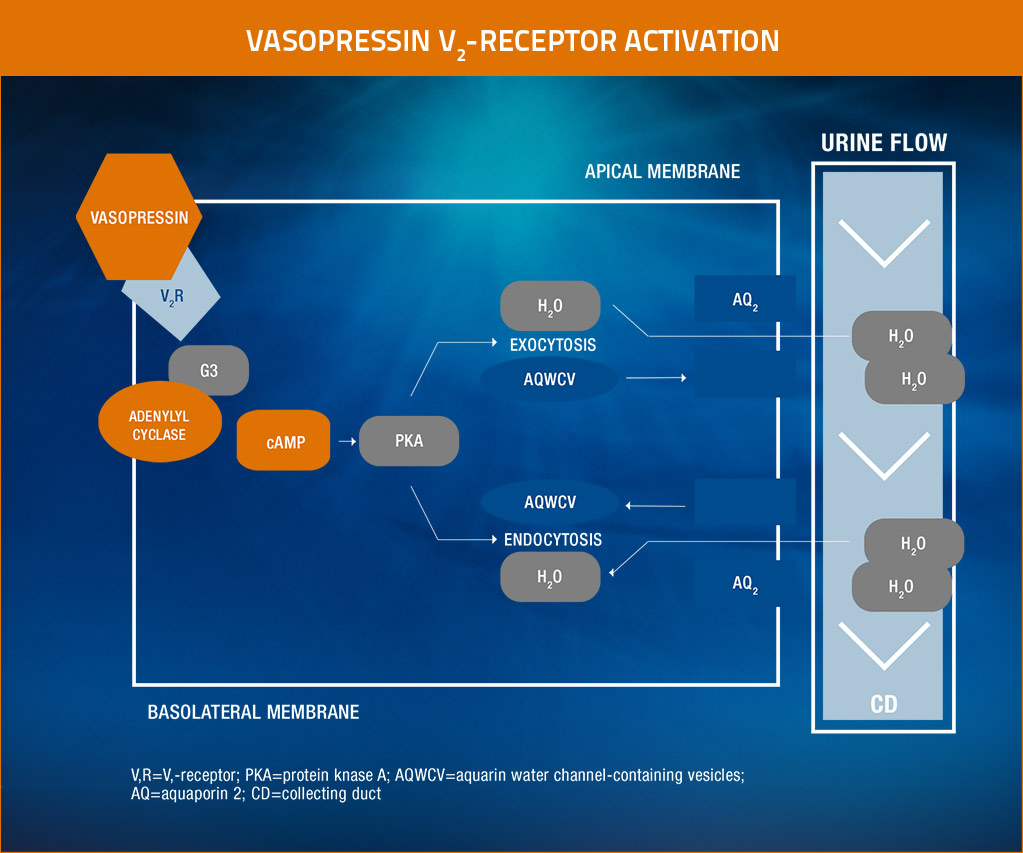
The antidiuretic effects of vasopressin occur when it binds to the V2-receptors

Vasopressin contributes to water homeostasis by promoting the reabsorption of fluid through the V2-receptors by binding to these receptors, causing the translocation of aquaporin-2 water channels to the apical membrane in the collecting duct of the kidney.3,4
The diagram below illustrates the mechanism of antidiuresis by vasopressin.
Adapted from Finley, Circulation, 2008.
Vasopressin V2-receptor activation: The binding of vasopressin to the V2-receptor stimulates a Gs-coupled protein that activates adenylyl cyclase, in turn causing production of cAMP to activate protein kinase A. This pathway increases the exocytosis of aquaporin water channel–containing vesicles and inhibits endocytosis of the vesicles, both resulting in increases in aquaporin-2 channel formation and apical membrane insertion. This allows an increase in the permeability of water from the collecting duct.5
Relationship of vasopressin to hyponatremia
Inappropriately elevated plasma levels of vasopressin in conditions such as SIADH and heart failure increase water reabsorption and retention, which will disproportionately expand the plasma volume, thus resulting in dilutional hyponatremia. In patients with syndrome of inappropriate antidiuretic hormone (SIADH), vasopressin release is not fully suppressed, despite hypo-osmolality, owing to other causes, including ectopic production of vasopressin by some tumors. The persistence of vasopressin release due to nonosmotic hemodynamic stimuli is also predominantly responsible for water retention and hyponatremia with hypervolemia and edema-forming disorders, such as heart failure.3

The SAMSCA MOA video illustrates the relationship between vasopressin and hyponatremia, as well as the potential benefits of vasopressin V2-receptor antagonism.