SAMSCA® (tolvaptan): Effect on fluid balance vs placebo
Significant effect on serum sodium concentrations and fluid balance in patients with hyponatremia on day 1*†
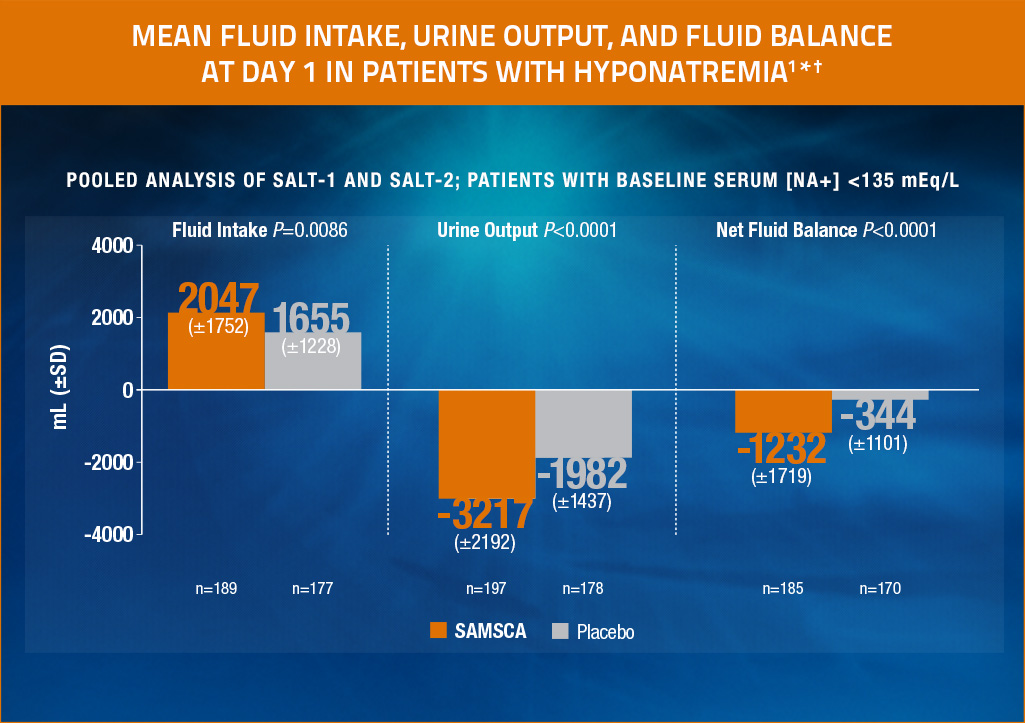
- With SAMSCA, urine output was greater than fluid intake, which results in a net negative fluid balance1
Mean fluid intake, urine output, and fluid balance at day 1 in patients with hyponatremia1*†
† Fluid balance is equal to total fluid intake (oral or IV) minus urine output. Only subjects whose time spans in both urine collection and fluid intake were no less than 22 hours and no more than 26 hours are included.
* Fluid balance was a secondary end point. Primary end point from pivotal clinical trials was average daily AUC for change in serum sodium from baseline to Day 4 (SAMSCA, 4.0 mEq/L; placebo, 0.4 mEq/L [P<0.0001]) and baseline to Day 30 (SAMSCA, 6.2 mEq/L; placebo, 1.8 mEq/L [P<0.0001]).2
† Fluid balance is equal to total fluid intake (oral or IV) minus urine output. Only subjects whose time spans in both urine collection and fluid intake were no less than 22 hours and no more than 26 hours are included.
- Greater than 1 L net fluid output in patients treated with SAMSCA® (tolvaptan) vs placebo1
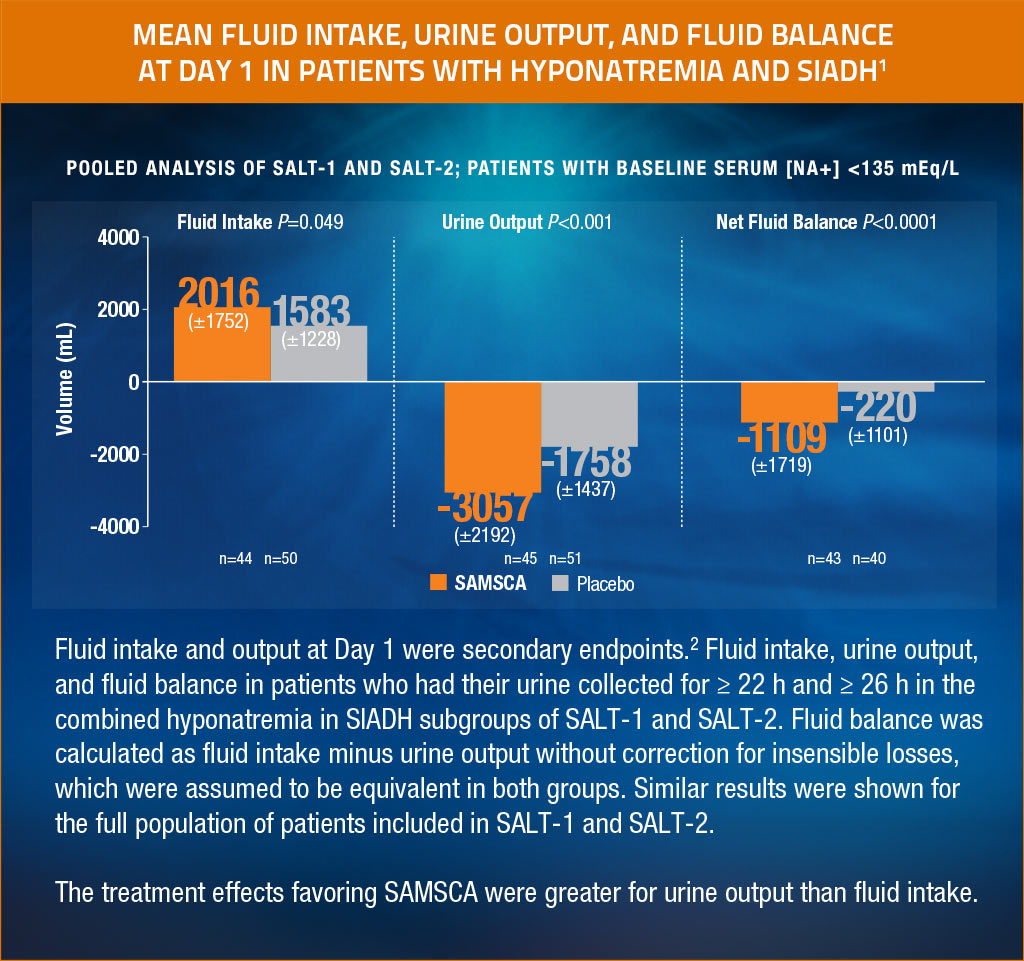
Hyponatremic SIADH patients treated with SAMSCA lost significantly more net fluid over the first study day than those on placebo
Adapted from Verbalis et al, Eur J Endocrinol, 2011.3
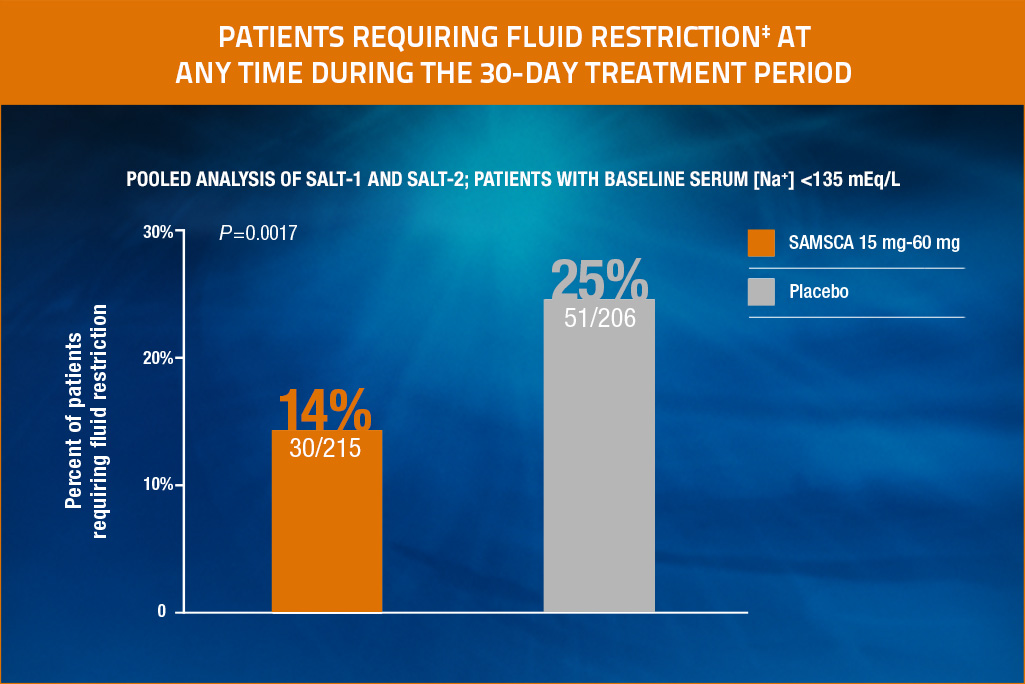
Reduced need for fluid restriction with SAMSCA
With SAMSCA, the percentage of patients needing fluid restriction (<1 L/day) at any time during 30-day treatment period‡ was significantly less (P=0.0017) than with placebo.
‡Fluid restriction was to be avoided during the first 24 hours of therapy.
Significantly fewer patients required fluid restriction during treatment with SAMSCA in the SALT trials (14% [30/215] vs 25% [51/206] for placebo), P=0.0017.
Fluid restriction during the first 24 hours of therapy may increase the likelihood of overly-rapid correction of serum sodium and should generally be avoided.
Too-rapid correction of serum sodium (e.g., >12 mEq/L/24 hours) can cause serious neurologic sequelae, including osmotic demyelination syndrome (ODS).

The SAMSCA MOA video illustrates the relationship between vasopressin and hyponatremia, as well as the potential benefits of vasopressin V2-receptor antagonism.