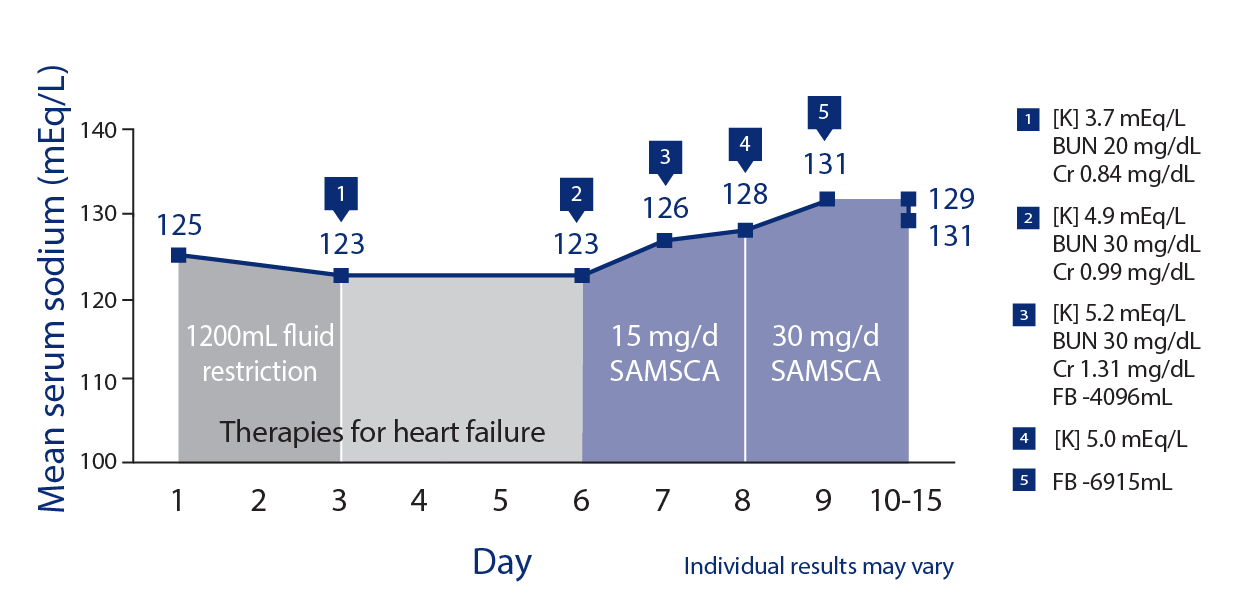
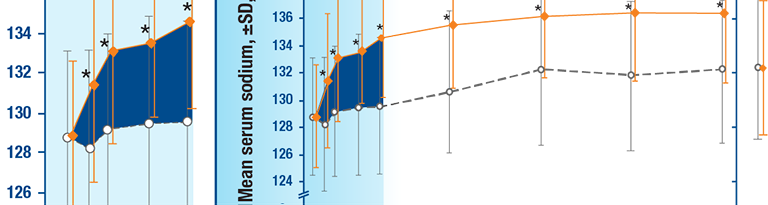
Young adult male with hyponatremia and congestive heart failure
- Congestive heart failure
- Admission serum [Na+]: 125 mEq/L
- No improvement in serum sodium despite fluid restriction
Patient image is fictional. Case is based on an actual patient. However, some information has been modified to simplify the presentation. Individual results may vary.

SAMSCA offers once-daily oral dosing with the flexibility to titrate